Latest News
This is a new regular column where readers can submit generic (not specific project) questions relating to corrosion protection, to be answered by corrosion experts. This month, the questions relate to zinc rich coatings and the problems of monitoring CP on pipelines affected by induced AC
Question:
Zinc-rich primers are commonly used for the protection of structures exposed to severe environments. The level of zinc dust is classified by the weight of zinc in the dried film, and most standards and specifications require at least > 77% to meet the performance demands. In conventional zinc-rich epoxy primers the high levels of zinc are achieved by adding large amounts of zinc dust particles into an epoxy matrix and the dispersion of this is critical to ensure electrical continuity and hence galvanic protection of steel.
I have heard of zinc epoxy primers on the market with considerably lower zinc levels (as low as 31% by weight). Do these primers still provide good galvanic corrosion protection and maintain good adhesion and mechanical properties of the dried film ? PF
Answer:
Corrosion protection paints using metallic zinc dust as a protective pigment have been used successfully for many decades. Zinc levels in coatings are classified by various standards bodies; common examples being:
• ISO 12944 = “zinc rich” >80% (Zinc dust on dry film)
• BS5493 = “zinc rich” >95% (Zinc dust on weight of pigment)
• BS4652 = “zinc rich” >85% (Zinc metal on dry film)
• SSPC Paint 20 Specification
Type 1 – Inorganic zinc rich paints (Zinc silicates)
Type 2 – Organic zinc rich paints (Epoxy or other organic binders)
• “zinc rich” Level 1, ≥85% (Zinc dust on weight of dry film)
• “zinc rich” Level 2, ≥77% to <85% (Zinc dust on weight of dry film)
• “Reduced zinc” Level 3, ≥65% to <77% (Zinc dust on weight of dry film)
Metallic zinc can protect ferrous substrates via several mechanisms:
• Galvanic protection – Fundamental physical chemistry dictates that if two different metals are in intimate electrical conductive contact, the more reactive metal will form the anode in a corrosion cell and preferentially dissolve to protect the less reactive cathodic species. As zinc sits above mild steel (principal component being iron) in the electrochemical series then metallic zinc in contact with steel will react preferentially, and sacrificially, to protect the steel. In the case of zinc rich primers, the paint is formulated with a very high pigment to binder ratio (close to or even at the critical PVC) such that metallic zinc particles are in intimate contact with each other within the dry film and with the steel substrate at their interface. For a galvanic mechanism to work properly, it is important that the substrate is blast cleaned to a high standard, (typically minimum Sa2½ ISO 8501-1), with enough surface profile to give a good mechanical key. The zinc salts formed as a result of the dissolution of the zinc dust at the anode of the corrosion cell can build up and plug the gap in the coating thereby providing a self-healing effect which can close off the corrosion cell and give a longer-term barrier protection. For true metal-to-metal contact within the coating, typically a level of at least 77% zinc on dry film would be specified. These primers can be designated “Zinc Rich”.
• Barrier effect – On exposure to the atmosphere the zinc metal will react to form zinc salts on a localised basis within the dry film which will plug any porosity within the coating and provide excellent barrier protection.
• Local effect on pH – The zinc salts formed at a corrosion site, in addition to providing a physical barrier to protect the substrate, are inherently alkaline in nature thereby raising the pH at the corrosion site. High pH conditions do not favour the electrochemical corrosion mechanism and therefore the rate of corrosion is suppressed.
Protective primers can be formulated using lower levels of zinc dust than those defined as “zinc rich”. These products must be carefully formulated – as with zinc rich primers these are typically at a high pigment to binder ratio, using a suitable combination of filler pigments so that the zinc dust particles are not totally encapsulated by the binder and therefore still available to react with the external environment. In the case of these reduced zinc primers, there is insufficient zinc to give true galvanic protection or extensive zinc salt formation and plugging of large corrosion cells, as in extremely corrosive environments such as offshore and marine. These products can however provide effective protection in less demanding environments by means of their barrier and pH buffering mechanisms as described above.
There is no official minimum level of zinc in a reduced zinc primer, although the performance levels will need to be carefully assessed to meet the appropriate specification requirements. Very thin film (~15µ dft) inorganic weldable primers are used on a widespread basis in production of steel stock. These will require very low levels of zinc (sometimes around 15%) in order to meet the required low levels of zinc fumes generated by subsequent welding processes. These products are only designed for temporary protection prior to fabrication.
Formulation of an effective zinc rich or reduced zinc primer requires a high degree of effort (or luck!) on the part of the formulator – It is not just a case of putting in a high loading of zinc and hoping for the best. The higher the zinc loading, then various factors such as poor application properties, weak, powdery film, poor adhesion and importantly, higher cost will have to be considered. A good zinc rich or reduced zinc primer will provide the correct balance of performance in the designated environment, good spray characteristics, good film properties, compatibility with subsequent coats of paint, all for an acceptable price – Not an easy task! MM
Latest News
Job Title: National Corrosion Service Helpline Manager
Location: Remote working
Company Name: Institute of Corrosion (ICorr)
Job Type: Part-Time
Applications: via email to admin@icorr.org, attaching CV
Closing Date: 24 April 2020
Job Description:
- To act as the first point of contact for technical enquiries received by the National Corrosion Service (NCS)
- To refer each enquiry to an appropriate technical expert based on their expertise, availability and track record of addressing enquiries to the satisfaction of the customer
- To resolve each enquiry in a professional and timely manner
- To maintain and update a database of available technical experts across a range of corrosion-related disciplines
- To keep an auditable record of the number of enquiries received, the manner of their resolution and their final outcome
- To liaise with colleagues within ICorr to promote the NCS through appropriate channels and where possible seek to highlight successful case studies
Requirements
- Professional experience (preferably at senior level) in the practice of corrosion management and control
- Experience in fielding technical enquiries from industry
- Prior knowledge of where to access technical expertise across a range of corrosion-related disciplines
- Availability on a part-time basis (typically less than one day per week)
- Working knowledge of Institute of Corrosion activities would be advantageous
Latest News
This series of features in Corrosion Management intends to highlight industry wide engineering experiences, practical opinions and guidance to allow improved awareness for the wider public, and focused advice to practicing technologists. The series is prepared by ICorr Fellows who have made significant contributions to the field of corrosion management with superlative past industry involvements. The first contribution in this series is “ Metallic Materials Optimisation in Hydrocarbon Production Systems”, by Bijan Kermani, FICorr.
Metallic Materials Optimisation in Hydrocarbon Production Systems
Hydrocarbon producing facilities are potentially subject to both external and internal corrosion threats: in the case of the former, from hostile and geographically remote operating environments, and in the latter from the presence of wet produced fluids and acid gases. Both of these threats continue to impact materials selection, engineering design and through life integrity management. Selection and optimisation of appropriate materials, which can tolerate given production scenarios together with effective whole life corrosion management, remain key operational challenges and underpin successful hydrocarbon production, economy, safety and security. Correct choice of materials and their optimisation in such systems at the design stage is therefore an essential element of an effective corrosion management programme to achieve high reliability and trouble-free operation. The choice is governed by a number of principal parameters including adequate mechanical properties, corrosion performance, joining integrity, availability and cost.
This Fellow’s Corner gives an outline of a materials optimisation strategy by combining a number of these key ingredients. It takes advantage of materials with proven track record while describing attributes essential for such a holistic approach. It focuses on internal corrosion by produced and injected fluids as the principal criteria in materials selection. While descriptive, a basic of knowledge of materials and corrosion is nevertheless, highly advisable so that a fit for service solutions is achieved.
Amongst the parameters outlined above, two elements are elaborated further.
i. Corrosion Threats: given the conditions associated with hydrocarbon production and that of gas/water injection, internal corrosion must always be seen as a potential risk. The risk becomes real once an aqueous phase is present and able to contact the material, providing a ready electrolyte for the corrosion reaction to occur. The need to reliably handle wet hydrocarbons arises from the increasing number of fields where significant levels of CO2 and H2S are present under more arduous operating conditions. In addition, the growth in the need for increased production which invariably entails water and/or gas injection to maintain reservoir pressure and/or enhance recovery can introduce O2 and the potential for microbiological activity which presents a different type of corrosion threat.
While most classical forms of corrosion are encountered in hydrocarbon production, the principal types where the majority of failures occur remains limited. The most prevalent types of damage encountered include metal-loss corrosion and localised corrosion manifested in the presence of CO2 (sweet corrosion) and H2S (sour corrosion) dissolved in the produced fluids and by the presence of dissolved oxygen in water injection systems. These three types of corrosion threat should be addressed specifically in the material optimisation process when assessing corrosion risk. In addition, the potential risk of environmental induced cracking needs to be addressed effectively.
ii. Metallic Materials: The oil and gas industry sectors continue to lean heavily on the use of carbon and low alloy steels (CLAS) which are readily available in the volumes required and able to meet many of the mechanical, structural, fabrication and cost requirements. The technology is well developed and for many applications these materials represent an economical choice. However, the inherent corrosion resistance of CLASs is relatively low. Consequently, their successful application invariably requires combination with one or more whole-life forms of corrosion mitigation against both internal and external exposure conditions.
A materials optimisation strategy requires an integration of all the above key parameters to allow the selection of the most suitable, safe and economical material option and corrosion control procedure. The parameters in such a strategy should take advantage of two key elements of trusted/proven methods reflecting past experience as well innovative solutions. Considering the above elements, the methodology described here has adopted a stepwise process by first exploring the feasibility of using CLAS followed by its use in combination with corrosion inhibition (CI) and/or a corrosion allowance (CA). If this approach is not feasible, then corrosion resistant alloys (CRAs) need to be considered, all based on whole-life costing.
Governed by system corrosivity and the feasibility of corrosion mitigation measures, materials are selected accordingly. It is important to note that while CLASs are chosen primarily according to their general and localised metal loss corrosion resistance, with adequate resistance to different types of H2S induced cracking, CRAs are normally selected primarily based on their resistance to environmental induced cracking. This latter threat includes sulphide stress cracking and chloride stress corrosion cracking or a combination thereof, as affected by the operating temperatures and conditions. The exception for CRAs is under extreme conditions – typically a combination of high temperature, low pH, high CO2 and H2S – where general corrosion may also, or exclusively, have to be considered in the overall selection strategy.
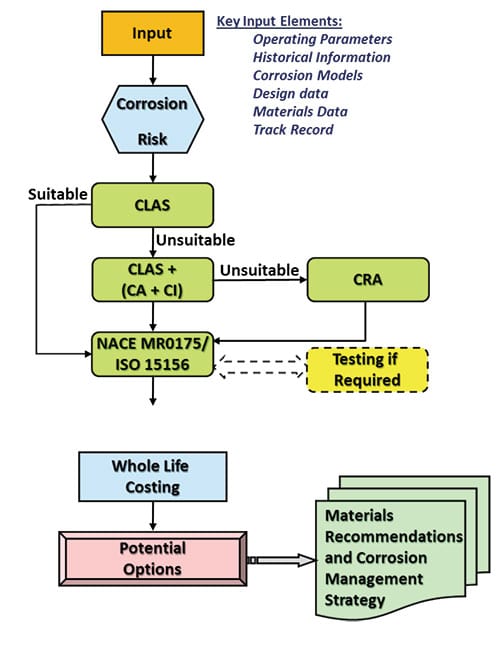
The simple overall approach to the optimisation strategy, shown in the figure, captures these necessary steps in finalising the materials choice. This simplified roadmap includes several key elements taking on board and incorporating (i) corrosion risk evaluation, (ii) operating conditions, (iii) corrosivity assessment, (iv) erosion velocity, (v) window of application of individual alloy, and last but no least (vi) whole life costing of potential materials options and corrosion mitigation methods. The simple methodology is based on utilisation of past successes and lessons learnt in effective use of CLASs and integration of key parameters to allow the selection of the most suitable, safe and economical material option and corrosion control measures.
The roadmap follows a methodical route to highlighting options and the most appropriate and cost effective materials, and outperforms similar models through the unique integration of key parameters. The strategy is applicable to optimisation of materials for all applications including downhole completions, surface and transportation facilities.
A distinction should be made here between materials used for subsurface (wells), where welding and CA may not be applicable, in contrast to materials for above surface facilities (subsea, topside or transportation) where corrosion mitigation in the form of CI deployment, or CA, become feasible.
Throughout the process, in the absence of reliable data, a methodical approach to performance evaluation needs to be put in place and be implemented. This provides a flexible structure to allow realistic testing to enable input of complementary data to provide further confidence on their application.

Latest News
Mitigating corrosion of embedded rebar is a perennial problem for reinforced concrete structures, requiring preventative maintenance and repair. Topical corrosion inhibiting treatments require a clean, properly prepared surface area to work as intended, however according to the company, Cortec’s new MCI®-2020 Gel is designed to work when proper surface preparation cannot be achieved, or is economically undesirable, by delivering its “Migrating Corrosion Inhibitor”™ technology directly to the depth of reinforcement.
The gel is an injectable corrosion inh ibitor that provides a robust dose of corrosion protection directly where it is most needed. Once inside the concrete, the inhibitor can also move laterally through the concrete along the embedded reinforcement via liquid and vapour diffusion. The corrosion inhibitor molecules deposit on metal surfaces, forming a molecular layer that acts as a barrier to corrosive elements such as chlorides and from carbonation.
It is considered a mixed inhibitor that protects against corrosion at both the anodic and cathodic areas of a corrosion cell. Unlike some of its competitors, it does not contain secondary amines or nitrites, which together can form carcinogenic nitrosamines. It is also non-flammable and non-combustible, and can be used in almost any concrete repair or maintenance application, concluded the company.
Latest News
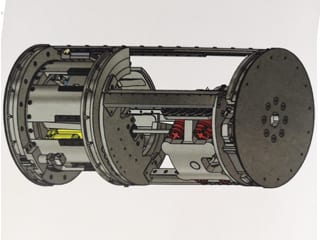
Forth Engineering, a Cumbrian engineering company is leading the development of a welding robot to repair pipelines from the inside. It is being described as a ‘world-first pipeline technology that will revolutionise performance and safety in industries around the world’.
The FSWBot is a friction stir welding robotic crawler for internal repair and refurbishment of pipelines, which is being developed by a consortium led by Forth Engineering in Cumbria with UK government backing. The consortium includes TWI, J4IC, Innvotek and London South Bank University. The development project is due to be completed by end of January 2021.
According to the company, the FSWBot is envisaged to be a five-segment or six-segment PIG type vehicle which will be inserted at the production end of an oil pipeline and will travel with the oil flow to a pre-designated spot to perform a repair. One segment will carry the FSW machine and a steel patch dispenser, with the other segments carrying the navigation, control system, communications, non-destructive testing (NDT) and power storage/generation payloads.
On entering the pipe segment containing the pre-identified defects, the robot will stop, then slowly advance until the FSW system is in place over the defect. It will then lock itself in place and confirm that it is correctly located to perform the repair.
An onboard turbine in a duct within the robot will harvest energy from the oil flow within the pipe to augment any power cells carried on the system, with the duct providing through flow in the pipe.
Once energised, the FSW unit will deploy a milling tool to cut away the corroded area and prepare a pocket in the pipe wall into which a steel patch will be dispensed.
The FSW unit will then weld this patch in place and deploy the milling system again to ensure that the patch is flush with the pipe wall and will not initiate turbulent flow, nor impede the passage of subsequent cleaning or inspection PIGs.
FSWBot will then deploy NDT packages to inspect the weld for quality assurance before unclamping and moving downstream to repeat the process on any further defects.


