
Industry News
The PDA Europe Conference & Exhibition will take place on 17-18 November 2022 in Porto, Portugal.
After almost two years of online events as a consequence of the Covid-19 pandemic, the organising committee are looking forward to welcoming you in-person for an exceptional two-day gathering in the beautiful region of Porto.
Registration for this is now open. For further information, including exhibit space and hotel accommodation,
see: www.pda-europe.org


Industry News
Geothermal energy could be the “invisible key” to unlock new energy sources and help meet net zero greenhouse gas emissions by 2050, according to Queen’s University Belfast researchers.
Professor Mark Palmer and Joseph Ireland from Queen’s University Belfast have been working on a new landmark report for the Department for the Economy and the Northern Ireland Geothermal Advisory Committee.
The report, “Net Zero Pathways: Building the Geothermal Energy Sector in Northern Ireland”, highlights that
building the geothermal energy sector can help transition Northern Ireland towards a low-carbon future and create an emerging market.
Future geothermal energy use is considered key in decarbonising Northern Ireland’s heat sector as it is a clean and naturally occurring source of energy. It uses the natural subsurface as a source of heat and has the potential to provide cooling and seasonal storage of energy.
Launched as part of Northern Ireland Geothermal
Energy Week, the Queen’s University report offers
detailed recommendations for the way forward and f
ocuses on the confidence-building actions needed to
unlock the opportunities for energy from geothermal
heating and cooling.

Fellows Corner
This article will look at the Electrochemical Noise Method for corrosion monitoring. Firstly, what are its attractions?
Well, it’s as unintrusive as its possible to get, just using the fluctuations which are produced naturally by the corrosion process to tell us the corrosion rate and also, by looking at the plots, the corrosion processes (the degree to which it affects the item being measured is maybe as little like the effect on the star of using the James Webb telescope to obtain its distance based on the red shift!). Secondly the measurement is quick (a few minutes) and interpretation of results is intrinsically simple. It can operate on battery power and the equipment is portable and with appropriate sensing electrodes it can be used for continuous monitoring. The remainder of this article gives examples of how results can be obtained using the ENM technique, not just from organic coatings, but also from reinforcing bars in concrete, and to screen inhibitors. The inhibitors work is part of an ongoing programme at Nottingham university and the other two pieces of work were done by students in Northampton.
First how does the electrochemical noise arise, and what do the results look like?
Rn= v/ I 1
Where Rn is noise resistance,
v and I are the standard deviations of voltage and current values, respectively, measured during a given time period is given by:
V2 = Vj−Vm)2/(n−1) 2
I 2= Ij−Im)2/(n−1) 3
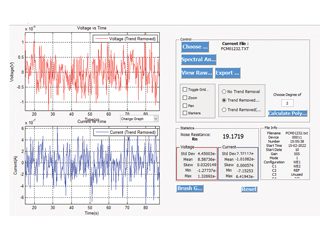
In equations 2 and 3, Vj is the voltage value measured at the jth time interval, Vm is the mean voltage in the given period of time, Ij is the current value measured at the jth time interval, Im is the mean current in the given period of time, and n is the number of time intervals. [1] For coatings, Rn equates to the DC resistance (Rdc), and EIS (0.1 Hz impedance). [2] There is evidence from this work and others that when there is significant corrosion rate, Rn relates to the value Rp obtained from Linear Polarisation Resistance (LPR). The analysis software creates a value of Rn, and typical ENM data are shown in Figure 1.
The first application of the ENM method described is to an anti-corrosive organic coating system.
The actual Noise arrangement used is known as the single substrate (SS) method, diagrammatically shown in Figure 2. You need two working electrodes for noise measurements, and with the single substrate arrangement you can have these as two areas of painted substrate on the same panel, isolated in lab work, by being contained in cells (but in site work, a dry piece of coated steel with a Calomel electrode in each cell, is adequate). The third electrode (reference) is the panel itself. When you examine an organically coated metal, the noise signal tends to be attenuated by the ionic resistance of the coating, and you end up with that resistance being dominant. In this example, the coating was applied to Q panels, cells were attached, and the coated area within each cell was exposed to 0.1 M chloride solution for several months (with occasional topping up). The ENM results were compared against DC resistance. The results are shown in the Figure 3. As can be seen, some areas of coating started with low values of resistance which tended to drop with time, while others had higher initial values, and these remained high. There was a strong correlation between the Rn values, the Rdc values, and the visual appearance. Also, the effects of thickness and number of coats were investigated. Thickness proved more critical than number of coats, although both were important, the higher the thickness the more protective the coating. The advantage of the electrochemical measurement over visual observation is, a) it tells you what is happening earlier, b) It indicates problems when you cannot see what is going on, c) it can be automated, and d) it gives you a number!
These advantages also apply in the case of the second example, that is measuring the corrosion rate of reinforcing bars in concrete. This is little more complicated to set up. The lab work was designed to be a precursor to taking the equipment out to site so non-glass electrodes were used viz solid-state silver /silver chloride solid electrodes, which worked just as well as Calomel electrodes. There is an ENM arrangement which can be applied to a coating or to concrete, which is the NOCS (No connection to the substrate) arrangement. This offers the attraction of being able to get a result without actually making any connection to the rebar itself. The experimental set up is shown in figure 4 and some typical results in Table 1. This work involved two sets of three bars in mortar, one of which contained no added chloride, and the other of which had 4% added chloride. The results were quite clear, the bars in the 4% were corroding (corrosion rate inversely proportional to Rn) many times faster than the bars in the 0% NaCl, although there was some minor variation between bars. Unlike the coating work, we cannot check these particular samples yet as they are still under test and have not been broken open. However previous published work [3] showed beyond reasonable doubt that the Rn value correlated very well to the subsequently observed corrosion.
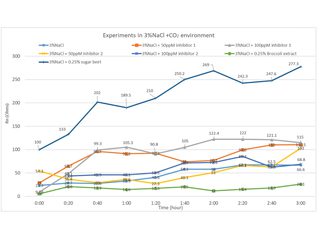
The third example is testing inhibitors This was driven by the wish to test green inhibitors and see if they could be used as alternatives to conventional more toxic inhibitors such as propargyl alcohol. The application considered was the need to inhibit corrosion of the steel pipes used to carry the CO2 saturated oil, containing some sea water. The experimental set up is shown in Figure 5. Two small nominally identical rectangular steel samples were contained in a sample holder made employing the ‘additive manufacturing’ process (a form of 3D printing). In this case it was from an ABS polymer powder. The black blanking-off compound was provided by an anticorrosion coating manufacturer. It was found essential to stir the solution to get reproducible results, although in practise a significant flow rate is likely. Some results are shown in Figure 6, where a particular green inhibitor (sugar beet) proved more effective than conventional inhibitors. The lab investigation compared the ENM method both against corrosion loss by ICP-MS analysis, and also against the more commonly used LPR method. This work has not yet appeared in a journal publication. But a paper has been submitted to EUROCORR 2022.
This has been whistle stop tour showing the application of this ENM technique to three different fields. If anybody wants to find out more, get in touch with the author.
1) Yang, L. and Chiang, K.T. (2010) On-line and real-time corrosion monitoring techniques of metals and alloys in nuclear power plants and laboratories. Understanding and Mitigating Ageing in Nuclear Power Plants., pp. 417-455
2) Comparison of ENM, EIS and DC Resistance for Assessing and Monitoring Anti-Corrosive Coatings Douglas J Mills JCSE 2000
3) Mills, D.; Lambert, P.; Yang, S. Electrochemical Noise Measurement to Assess Corrosion of Steel Reinforcement in Concrete. Materials 2021, 14, 5392. https:// doi.org/10.3390/ma14185392
Acknowledgements
The author would like to acknowledge the assistance of Paul Lambert relation to the concrete project, and extend his thanks to students: Tian Yang Lan, Reuben Osahon and Chiata Collins for permission to publish some of their results. And to DCVG company for providing The Noise Measuring Equipment.
Douglas J Mills

Figure 1: Typically Noise plots of current and voltage against time.
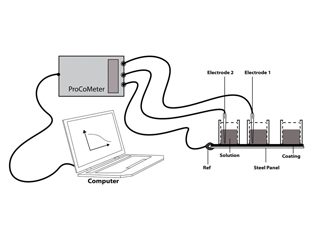
Figure 2: Schematic diagram showing how ENM is applied to coated metal (Single Substrate arrangement) (Solution is typically 0.1 M NaCl).
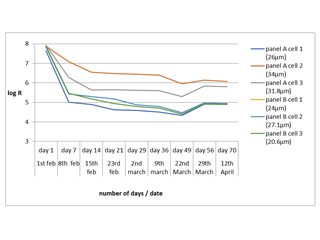
Figure 3: Typical results obtained from Hammerite coating of Resistance against time.
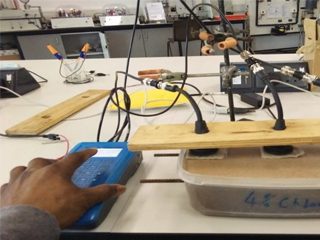
Figure 4: Set up for measuring Rebar in concrete using NOCS arrangement of ENM.

Fellows Corner
This series of articles is intended to highlight industry wide engineering experiences, practical opinions, guidance, and focused advice to practising technologists. The series is written by ICorr Fellows who have made significant contributions to the field of corrosion management. The articles in this issue feature contributions from David Harvey, who gives a personal view of his career as a senior CP Engineer, and Douglas Mills, who discusses the use of Electrochemical Noise Measurement in determining corrosion.
A Career as a Cathodic Protection Engineer
This article highlights some aspects of an interesting and rewarding career in the cathodic protection industry and with the Institute of Corrosion. It aims to show what can be experienced within the workplace and outside of it, in a varied, fascinating and satisfying career over 50 years gained with consultants, oil and gas operators, cathodic protection companies and engineering design houses. Hopefully this will encourage newcomers to the industry that there is great career to be had in cathodic protection.
I, like many others, came into cathodic protection by accident. I joined a CP Specialist Consulting Engineering Company as a draughtsman while still completing my day-release HNC in Electrical Engineering. I was fortunate to have an excellent mentor in David Lewis, former ICorr President, who gave me John Morgan’s “Cathodic Protection” textbook to study. I progressed on to become a CP design engineer. As I did not drive at that time and site visits were limited, I benefitted from picking the brains of my colleagues’ knowledge and site experience, as back then, junior engineers were teamed up with a senior engineer to learn their trade, which regrettably does not happen today. However, one of the enlightening aspects about the CP Industry is that senior engineers are generally very open in sharing their knowledge and experiences – both good and bad – to enable us to continually improve the way we approach projects and produce more cost effective, efficient designs.
CP Engineers come from many different disciplines (e.g. mechanical, electrical, chemical, civil etc) some from grass roots and others from universities. The application of CP to structures requires detailed interaction with all disciplines so one ends up knowing a little about a lot, rather than a lot about a little, unless specialising in one particular area. Rarely are two projects identical as there are so many different parameters to be considered. Today, there are training courses, exams and certification offered by ICorr at all levels from Tester to Senior Design Engineer (Levels 1- 4 of ISO 15257). However, CP is something you also need to learn from field experience – often by trial and error. It is not something you can just study and apply effectively from a desk alone. Design spreadsheets can often contain errors or have bad data inputted so the output needs strong experience to know if it is sound or “rubbish”.
My practical experience was initially gained by secondment to Middle Eastern oil companies. It covered applying cathodic protection to oil and gas fields, pipelines, associated tankage, and marine facilities. This was a very exciting time for a young man who had not previously been out of the UK. Working in the desert oil fields was almost like being on Safari. On return to UK, I was appointed a CP project engineer. Over time, I moved up to Engineering Manager and Consultant in a number of cathodic protection companies, design houses and as an independent consultant.
I was responsible for all aspects of sacrificial anode and major ICCP systems for pipelines, refineries/petrochemical facilities, and inshore/offshore marine structure projects.
My career as a cathodic protection engineer has given me the opportunity to visit and work in more than 25 different countries and meet many exceptional people at all levels. Occasionally, one could find sufficient spare time to visit some of the local tourist attractions, e.g. various roman ruins, Great Wall of China, Great Pyramids, Panama Canal etc. which were very enjoyable benefits I would not have otherwise gained.
ICorr Activities
As the industry expanded in the 1980’s, I became involved in ICorr activities when they had the Joint Venture with NACE (CCEJV), which became the Corrosion Engineering Association (CEA).
Initially, I joined a CCEJV cathodic protection work group to assist with the preparation of a State-of-the-Art Report to be published by ICorr and NACE. This enabled me to learn from my peers and, at the same time, put some of my experience back into the industry for the younger engineers to benefit from. Later, I became Work-Group Chair, then Task-Group Chair. I was then appointed Technical Activities Coordinating Committee (TACC) Chair. This role also included being the Conference Programme Manager for “UK Corrosion” which was a major three-day event in the corrosion world 1987-1991. As TACC Chair, I also attended NACE Committee Weeks and Corrosion Conferences in USA as the UK ICorr/CCEJV representative. This was a tremendous opportunity for interaction with cathodic protection engineers and manufacturers from around the world. Regrettably, after much good work, NACE and ICorr parted company in 1988 and the CEA became the Corrosion Engineering Division of ICorr.
I also became the representative for Pipeline Industries Guild and the Institute of Petroleum on the BSI CP GEL 603 committee in 1985 which was revising the UK CP bible, BS CP1021. This was eventually published as BS7361 in 1991. In 1993 I was elected to chair this committee, a post I held for the next 19 years, coordinating the UK input into numerous BS/CEN CP standards prepared and published during this time.
Apart from the BSI committee, I also became involved in various ICorr committees and Council:
• Member of Council 1998-Present.
• Chair of Professional Assessment Committee 1998 – 2012.
• Chair of CP Certification Sub-Committee 2006-2019.
• Chair of Course Approvals Board 2013-2019.
• Member of CP Governing Board (2002- 2020).
• Member PDTC Committee (1998-2020).
• QA Advisor attaining ISO 9001 Certification.
• ICorr Representative for UK to CEOCOR (2008 – 2012).
• Professional Affiliate Engineering Council Coordinator with Society of Operations Engineers – current role.
• Primary author/updater of 5 ICorr CP training Courses to ISO 15257 for On-land and Marine Structures.
As a result of these activities, I was awarded Honorary Fellow Member of ICorr in 2018.
One of the other milestones in my career was attaining Professional Affiliate Status for ICorr with the Engineering Council. This was followed by the setting up a Registration Agreement with the Society of Environment Engineers enabling suitably qualified and experienced members to apply for registration with the Engineering Council as a CEng, IEng or EngTech. Having set the system up, I thought I should be the guinea pig to try it out. My application was successful and I was awarded CEng. With the demise of SEE, I set up a new Registration Agreement with the Society of Operations Engineers.
As a result, more than 50 of our members have become registered as Chartered Engineers – a tremendous benefit available for our professional members.
In Conclusion
As I look back over the last 50+ years, I can reflect on the many good memories of a varied career, the many friends and colleagues I have worked with and the opportunities I was given to expand my knowledge. I have tried to put back some of this for young engineers to consider that a career in CP can be very interesting and rewarding. To be honest, I still get satisfaction from it and in retirement, I am still dabbling, doing some CP design appraisals/approvals. A long and interesting, satisfying career in CP is very achievable albeit with a lot of hard work.
David Harvey CEng, FICorr(Hon)

A Cathodic Protection Engineer at work.



