In this issue the questions relate to monitoring of CP on pipelines and what causes amine blush on epoxy coatings. Readers are reminded to send in their technical questions for possible inclusion in the column in future.
Question:
I maintain a buried pipeline in an environmentally sensitive area and I wish to follow best practice in monitoring the CP system on it. I am thinking of fitting remote monitoring but I am concerned how accurate and reliable any reference electrodes that I use with a data logging system will be. Related to this I do not want to excavate to install the reference electrode to pipe depth, can I bury the reference electrode just below the surface near the existing Test Point? BK
Answer:
The practice you describe is one that is widely followed, for example in GRTgaz France on their high- pressure gas transmission system they use remote monitoring equipment from Italy and fixed reference electrodes often buried close to the surface under open bottomed surface boxes to aid their location and replacement. National Grid in the UK use remote monitoring extensively. However, there are a number of issues for you to consider:
1. Fixed reference electrodes can be significantly unreliable and their electrode potential can drift significantly from what you and the manufacturer may expect. This may be particularly so with electrodes in soils with significant groundwater movement. It is recommended that all fixed reference electrodes are supported with a method such as a UPVC tube from ground level to electrode level to permit a calibrated, portable reference electrode, likely a Cu/CuSO4 to be deployed close to the fixed electrode and the drift assessed. Depending upon how important the accuracy is, this should be done frequently.
2. Placing electrodes remote from the pipe will introduce errors in measurement, more in high electrical resistivity soils than in low, this is due to IR errors due to CP current flowing in the soil. The errors may be low with the electrode directly over the pipe and if your pipe coating quality Is high.
3. You might also consider deploying a steel ‘coupon’ that most remote logging systems will permit to be switched ‘OFF’ for Instant OFF measurements that should be more accurate in respect of IR errors in the soil. But if your reference electrode is in error these data will also be in error.
4. Large, simple, old fashioned porous pot Cu/CuSO4 reference electrodes are pretty reliable if properly constructed but there may be concerns regarding soil contamination with CuSO4. If you use these with coupons, the coupon should be remote (upstream of downstream along the pipe, not laterally spaced from the pipe) from the Cu/CuSO4 electrode to prevent CuSO4 leaching into the soil and plating out on the coupon. If this happens all the measured data will be wrong.
5. In France they consider that the values they measure in the way above to be accurate only to 50-70mV. So be cautious with data close to under or over-protection values if the data come from this sort of installation. B.Wyatt, Corrosion Control Ltd
In GRTgaz France, we have installed and used Remote Monitoring Systems (RMS) with fixed reference electrodes for about 8 years. They are installed in open bottomed surface boxes to be regularly compared to a portable reference electrode (during detailed and comprehensive assessment of CP by a CP operator in the field). As they are used for general assessment of CP, the requirement for their accuracy is lower than that for portable reference electrodes used in the field for detailed and comprehensive assessment of CP ±100 mV for a fixed reference electrode to be compared to ±20 mV for a portable reference electrode. To facilitate the evaluation of the accuracy of fixed reference electrode, GRTgaz France decided to install them in the open bottomed surface boxes as it is then easy to minimise the gap between both electrodes (fixed buried and portable) and to change the fixed reference electrode when required (which is very seldom, as we have had good performance from our reference electrodes!). Sylvain Fontaine, Cathodic Protection Expert, GRTgaz, Compiègne, France
Question:
What causes amine blush on epoxy coatings? PS
Answer:
Amine blush is a combination of three unwanted reactions between amines and moisture/carbon dioxide which lead to the formation of amine hydrates (with water), ammonium carbamates (with carbon dioxide) or carbonates (with carbonic acid). It is the latter of these which presents the biggest problem as the reaction is irreversible and leads to surface defects which are difficult to remove (carbonation). In epoxy coating systems this will normally be at the surface/air interface as a result of the amines in the curing agent. Since carbonation is essentially an acid/base reaction it is kinetically favoured over the amine/epoxy reaction which we would prefer. This is particularly problematic in application conditions of low temperature and high humidity where the amine/epoxy reaction is retarded even further, and we have high levels of carbonic acid.
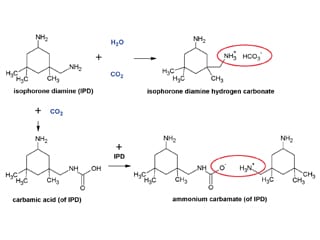
Figure 1. Reactions of isophorone diamine with H2O and CO2.
It has often been suggested that the way to resolve this would be to make the amine-epoxy reaction faster, but using more basic amines simply leads to more carbonation as they will more readily produce blushing via the acid/base interaction. It is interesting to note that in the past when the use of aromatic amine curing agents was more common there was no issue with blushing when using these products because aromatic amines are not basic. Of course, these products are now considered as toxic and as a result are no longer allowed in many parts of the world.
We can see from this that using less basic amines should therefore lead to improved blushing resistance, but they will also produce slow hardeners with long drying times. The question then becomes, how do we produce hardeners which will give the best combination of drying times and resistance to blushing? This is particularly relevant for solvent free systems where we have no drying advantage from the use of solid binders. Curing agent manufacturers have over time developed curing agents with improved resistance to blushing using various formulating methods. For example, forming amine adducts will improve the performance against bushing because the adduction process will remove some of the more basic amine functionality in the curing agent which is often responsible for blushing. It will also improve the compatibility of the amine with the resin thus reducing migration of free amine to the surface of the coating. Advances in curing agent development mean that today the best performing hardeners for blushing resistance in topcoats are based on formulated adducts of one or more amines.
Of course, epoxy resin and curing agent manufacturers can develop and test their resins and hardeners in combination as clear coatings, but the final paint will be formulated by coatings manufacturers where the fillers and additives used will also have an effect on the carbonation resistance of the end product. Even with the recent advances in curing agent performance, the end result will still depend on correct mixing and application of epoxy systems on site ensuring that the conditions in which the coating is applied meet with the manufacturers recommendations.
Stuart Darwen, CTP Advanced Materials GmbH, Germany



