
Ask the Expert
Why Do Bronze Medals Tarnish So Quickly?
By Roger Francis, RF Materials
Meet the Author
Dr. Roger Francis
Dr Francis has been a corrosion engineer for over 45 years. He has wide experience in the fields of marine corrosion, desalination, sour oil and gas corrosion, mineral processing, and the chemical and process industries. He has published over 100 technical papers in all these areas, particularly on copper alloys and duplex stainless steels. Roger has written seven books on various aspects of corrosion and has jointly edited three other books. The most recent was on corrosion in desalination plants. Dr. Francis has served on several standards committees working on corrosion testing of both copper alloys and stainless steels. In particular he was involved with the committee turning NACE MR0175 into ISO 15156.The author has served as chair of NACE Europe, two terms as the NACE (now AMPP) European Area Director and also as Chair of NACE STG 32 (Oil and Gas; metals). He was made a NACE Fellow in 2005 for his work in marine corrosion. In 2014 he received the T J Hull Award for his work in publications. In 2012 he set up his own corrosion consultancy business, RF Materials. In 2023, he received the Institute of Corrosion Paul McIntyre award presented to a senior corrosion engineer who, as well as being a leading practitioner in his field, has advanced European collaboration and international standards development.
Bronze has been used for a variety of medals for over two hundred years and given sufficient time without cleaning, they will all tarnish. Figure 1 shows an old coin with different degrees of tarnish on the two sides. Figure 2 is a bronze medal that also shows tarnishing.
There is a wide range of bronze alloys, where the chief alloying element is tin, aluminium, or silicon. The majority of bronze medals used to be made of LG2 gunmetal (Cu/5Sn/5Zn/5Pb) or something very similar. Tin bronzes are reddish, but not as strongly red as pure copper. Other elements may be added to modify the colour, for example, to make it more yellow. However, to cut costs, many modern “bronze” medals are actually brass (Cu/5Zn), which has a similar appearance to tin bronze but is not as corrosion resistant.
When a “bronze” medal is polished and shiny, the metal surface is very active and reacts readily in the air to form a very thin layer of cuprous oxide. This slightly dulls the appearance, but continued exposure to air will enable corrosion to continue, and a thicker layer of reddish brown cuprous oxide gradually forms. Although both alloys form cuprous oxide as the main corrosion product, the zinc or tin substitutes at some of the copper sites in the oxide matrix. Zinc makes the oxide layer less protective, while tin makes it more protective1. If the tin content is high enough, a separate layer of stannous oxide can form, but this is unlikely at 5% tin1. If a medal is exposed to an aggressive atmosphere for long enough, then a second corrosion product can form on top of the oxide layer, as shown in Figure 3. This is a basic copper carbonate, and it is less protective than the cuprous oxide layer.
Bronze has been used for a variety of medals for over two hundred years and given sufficient time without cleaning, they will all tarnish. Figure 1 shows an old coin with different degrees of tarnish on the two sides. Figure 2 is a bronze medal that also shows tarnishing.
There is a wide range of bronze alloys, where the chief alloying element is tin, aluminium, or silicon. The majority of bronze medals used to be made of LG2 gunmetal (Cu/5Sn/5Zn/5Pb) or something very similar. Tin bronzes are reddish, but not as strongly red as pure copper. Other elements may be added to modify the colour, for example, to make it more yellow. However, to cut costs, many modern “bronze” medals are actually brass (Cu/5Zn), which has a similar appearance to tin bronze but is not as corrosion resistant.
When a “bronze” medal is polished and shiny, the metal surface is very active and reacts readily in the air to form a very thin layer of cuprous oxide. This slightly dulls the appearance, but continued exposure to air will enable corrosion to continue, and a thicker layer of reddish brown cuprous oxide gradually forms. Although both alloys form cuprous oxide as the main corrosion product, the zinc or tin substitutes at some of the copper sites in the oxide matrix. Zinc makes the oxide layer less protective, while tin makes it more protective1. If the tin content is high enough, a separate layer of stannous oxide can form, but this is unlikely at 5% tin1. If a medal is exposed to an aggressive atmosphere for long enough, then a second corrosion product can form on top of the oxide layer, as shown in Figure 3. This is a basic copper carbonate, and it is less protective than the cuprous oxide layer.

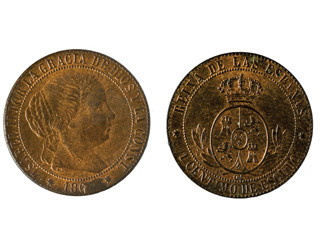

Figure 1: 19th Century Coin Showing Different Degrees of Tarnish on the Two Sides.
Figure 2: Tarnished Medal.
Figure 3:
Old Russian Coins from The Early 19th Century Showing Green Carbonate Corrosion Product.

Institute News
Prof. John Chudley,
Chair of the Engineering Council
www.engc.org.uk
The Engineering Council has signed a landmark Mutual Recognition Agreement (MRA) with the USA’s National Council of Examiners for Engineering and Surveying (NCEES).
The agreement was signed in Chicago by Laura Sievers, President of the NCEES and John Chudley, Chair of the Engineering Council. This significant event marks the first international agreement of its kind with any international counterpart in the NCEES’s 104-year history. This agreement establishes a streamlined process for recognising professional engineering qualifications between the UK and the USA and ensuring that engineers can practice across borders with greater ease and without compromising on professional standards.
As engineers are licensed at the state level in the US, participation in this agreement is determined by individual state licensing boards. Today, 26 US states have already confirmed their intent to participate in the agreement, marking a significant step forward towards wider adoption.
UK Chartered Engineers and US Professional Engineers wishing to use the streamlined route provided by the agreement will need to hold the International Professional Engineer (IntPE) title. To find out how to obtain this in the UK, see the Engineering Council website, and in the US see NCEES.
Source: Engineering Council Press Release
Contact: Helen Potts, Engineering Council – hpotts@engc.org.uk Tel: 020 3206 0568
Highlights of the MRA include:
-
Simplified Pathways for Certification: UK Chartered Engineers can now more easily obtain a US Professional Engineer license in participating states and US Professional Engineers can more easily achieve the CEng title in the UK.
-
Minimisation of Redundant Assessments: By recognising the equivalency of qualifications, the agreement removes the need for repeated assessments, thereby simplifying the certification process.
-
Increased Professional Mobility: The agreement opens new opportunities for engineers to work across the UK and the US, facilitating their professional growth and development.
-
Support for Key Industries: The agreement strengthens the engineering profession in both countries, supporting industries such as construction, automotive and aerospace.
-
Enhanced Trade in Engineering Services: By reducing restrictions on cross-border services provision, the agreement encourages the growth of international trade in engineering services.

Institute News
Our North-West (NW) Branch held its AGM on 1st October 2024 from 16:30 at Luther King
House, Brighton Grove, Manchester, a most interesting venue and an excellent conference facility.
The event included talks from Paul Lambert on 200 years of Cathodic Protection and Trish Conder on NDT in the renewable energies sector. An excellent buffet and social meet followed the presentations and formal business of the AGM.
Paul Lambert
Paul is Head of Materials and Corrosion Technology Team at Mott MacDonald, where he has worked for over thirty years. He is also a visiting professor at the Centre for Infrastructure Maintenance, MERI, Sheffield Hallam University. As Past President, he has a long-standing involvement with the Institute of Corrosion, where he currently provides support
in professional review and
assessment and is the current chair of the PAC committee. He has over 40 years’ experience in the performance, durability, corrosion, and protection of materials, including polymers. He has led on the research and development of investigation techniques and protective systems for plain, reinforced, and pre-stressed concrete and steel-framed structures, including cathodic protection and corrosion inhibitors. Additional roles include the preparation of expert evidence on coatings, road surfacing, concrete, corrosion, and stainless steels.
Dr Patricia Conder

Dr. Patricia Conder works as Principal Consultant at Inspection and NDT Consultancy, ESR Technology Ltd. Patricia has a multi-faceted background, having worked in a range of sectors, including electronics, chemicals, pulp, and paper, before entering the inspection and non-destructive testing field. Her current focus is the data science of inspection, from
planning and reporting to
evaluation of results combining to enable a greater insight into the underlying corrosion mechanisms. Patricia has an honours degree in physics from the University of Aberdeen and a PhD in materials science from the University of Birmingham.
Following the two excellent Technical Presentations, the Institute President – Stephen Tate thanked the North-West committee for all their hard work over the last 2 years in reactivating and growing the Branch. The Annual Branch AGM followed this, led by Greg Brown, who was unanimously elected as Branch Chair. The Chair presented a bottle of wine to long-serving and retiring committee member Brenda Peters, who will soon join the Trustees of the Institute of Corrosion as its new honorary secretary. Greg also thanked Kathleen Brook for all her work as University Liaison Officer during previous North-West Technical Sessions, including the recent highly successful Corrosion Awareness Day held in April at the University of Manchester. Kathy has now moved to Leeds to take up a new role there.
Upcoming Events
Following the AGM the branch will hold its annual Christmas dinner (date and venue to be concluded) before looking towards next year’s programme. The committee continues to grow, and if you would like to get involved or contribute towards an event, contact nwchair@icorr.org
For further details of NW technical programme, please visit: North West Branch – Institute of Corrosion (icorr.org) and scroll down to Local Technical Programme where copies of past presentations may be found.

Photo (Right): The Reception
Area at Luther King House.

Photo: Greg Brown –
Northwest Branch Chair Introduces Proceedings.

Photo: Professor Paul Lambert of Sheffield Hallam University and
Mott MacDonald.

Photo: Professor Paul Lambert Spoke Passionately on The Topic Of Protection Of Reinforced Concrete With Many
Case Studies Presented.

Photo: Dr Conder Discussed Various Case Studies Related to Deployment of Novel Ndt
Technologies With Particular Relevance To Emerging Net-Zero Energy Operations.

Uncategorized
The branch started the 24/25 season on Thursday October 10th with an in-person presentation by Ali Morshed on “Why is corrosion still a major integrity threat for many industries in the 21st century?” at the usual venue, the Lancaster Hall Hotel, Bayswater.
Ali Morshed holds PhD in corrosion engineering from University College London, MSc in corrosion engineering from Imperial College London, DIC and CEng.
He is the author of five corrosion management books and one MIC book with NACE/AMPP between 2012 and 2022. He is a corrosion engineer with more than 21 years of experience. Ali started his professional career in the oil and gas industry back in 2002, but since the introduction of the Morshed Corrosion Management Model (MCMM) in 2012 he has gradually expanded his work to many other industries.
Ali has worked in North Sea, North Africa, the Persian Gulf Region and South Asia. He provides corrosion management and MIC consultancy and training services for various industries.
A full description of this presentation will be reported in the next issue of Corrosion Management.
Upcoming Events
Readers are reminded of the ICorr 2024 YEP – Final Presentation of Case Studies, to take place on 21st November 2024,
15:30 – 21:00 at: Lancaster Hall Hotel, Craven Terrace, London, W2 3EL hosted by ICorr London Branch.
On December 5th, the branch will host its annual Christmas luncheon at the Royal Overseas League, St James’s, London, where the guest speaker will be Garry Richardson. Gary is a BBC Sports presenter, best known for his witty sports reports and interviews, and an experienced after-dinner speaker. He has presented Radio 4’s prestigious ‘Today‘ programme for
four decades.
For more details about sponsorship of this event or to book tickets, please contact Steve Barke at sjbarke@gmail.com
Photo: Ali Morshed, Independent
Corrosion Engineer.

Institute News
ICorr Central Scotland Branch will commence its 2024 – 2025 session on Wednesday, 30th October 2024. The session will prove to be very informative, with varied technical topics lined up. This follows an excellent response to the September call for presentation abstracts.
More abstracts / presentation topics are welcome. A mix
of in-person and hybrid events is being planned to allow presenters and attendees from other locations to benefit from the session’s program. The venue for meetings is INEOS Grangemouth HQ.
The Branch’s Committee is presently being constituted and
a branch AGM is planned; 3 positions have now been filled
and others are under review.
A further update will be made at council meeting of
6th November 2024.
Please contact Philip Enegela (philip.enegela@outlook.com) if you would like to serve on the Committee. A photograph will be published when the team is fully in place.
Photo: Dr Philip Enegela, Interim Chair Leading the Q and A Session of the Central Scotland Maiden Event on 27th June 2024.
