Ask the Expert
In this issue the questions relate to monitoring of CP on pipelines and what causes amine blush on epoxy coatings. Readers are reminded to send in their technical questions for possible inclusion in the column in future.
Question:
I maintain a buried pipeline in an environmentally sensitive area and I wish to follow best practice in monitoring the CP system on it. I am thinking of fitting remote monitoring but I am concerned how accurate and reliable any reference electrodes that I use with a data logging system will be. Related to this I do not want to excavate to install the reference electrode to pipe depth, can I bury the reference electrode just below the surface near the existing Test Point? BK
Answer:
The practice you describe is one that is widely followed, for example in GRTgaz France on their high- pressure gas transmission system they use remote monitoring equipment from Italy and fixed reference electrodes often buried close to the surface under open bottomed surface boxes to aid their location and replacement. National Grid in the UK use remote monitoring extensively. However, there are a number of issues for you to consider:
1. Fixed reference electrodes can be significantly unreliable and their electrode potential can drift significantly from what you and the manufacturer may expect. This may be particularly so with electrodes in soils with significant groundwater movement. It is recommended that all fixed reference electrodes are supported with a method such as a UPVC tube from ground level to electrode level to permit a calibrated, portable reference electrode, likely a Cu/CuSO4 to be deployed close to the fixed electrode and the drift assessed. Depending upon how important the accuracy is, this should be done frequently.
2. Placing electrodes remote from the pipe will introduce errors in measurement, more in high electrical resistivity soils than in low, this is due to IR errors due to CP current flowing in the soil. The errors may be low with the electrode directly over the pipe and if your pipe coating quality Is high.
3. You might also consider deploying a steel ‘coupon’ that most remote logging systems will permit to be switched ‘OFF’ for Instant OFF measurements that should be more accurate in respect of IR errors in the soil. But if your reference electrode is in error these data will also be in error.
4. Large, simple, old fashioned porous pot Cu/CuSO4 reference electrodes are pretty reliable if properly constructed but there may be concerns regarding soil contamination with CuSO4. If you use these with coupons, the coupon should be remote (upstream of downstream along the pipe, not laterally spaced from the pipe) from the Cu/CuSO4 electrode to prevent CuSO4 leaching into the soil and plating out on the coupon. If this happens all the measured data will be wrong.
5. In France they consider that the values they measure in the way above to be accurate only to 50-70mV. So be cautious with data close to under or over-protection values if the data come from this sort of installation. B.Wyatt, Corrosion Control Ltd
In GRTgaz France, we have installed and used Remote Monitoring Systems (RMS) with fixed reference electrodes for about 8 years. They are installed in open bottomed surface boxes to be regularly compared to a portable reference electrode (during detailed and comprehensive assessment of CP by a CP operator in the field). As they are used for general assessment of CP, the requirement for their accuracy is lower than that for portable reference electrodes used in the field for detailed and comprehensive assessment of CP ±100 mV for a fixed reference electrode to be compared to ±20 mV for a portable reference electrode. To facilitate the evaluation of the accuracy of fixed reference electrode, GRTgaz France decided to install them in the open bottomed surface boxes as it is then easy to minimise the gap between both electrodes (fixed buried and portable) and to change the fixed reference electrode when required (which is very seldom, as we have had good performance from our reference electrodes!). Sylvain Fontaine, Cathodic Protection Expert, GRTgaz, Compiègne, France
Question:
What causes amine blush on epoxy coatings? PS
Answer:
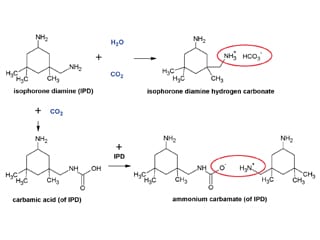
Amine blush is a combination of three unwanted reactions between amines and moisture/carbon dioxide which lead to the formation of amine hydrates (with water), ammonium carbamates (with carbon dioxide) or carbonates (with carbonic acid). It is the latter of these which presents the biggest problem as the reaction is irreversible and leads to surface defects which are difficult to remove (carbonation). In epoxy coating systems this will normally be at the surface/air interface as a result of the amines in the curing agent. Since carbonation is essentially an acid/base reaction it is kinetically favoured over the amine/epoxy reaction which we would prefer. This is particularly problematic in application conditions of low temperature and high humidity where the amine/epoxy reaction is retarded even further, and we have high levels of carbonic acid.

Figure 1. Reactions of isophorone diamine with H2O and CO2.
It has often been suggested that the way to resolve this would be to make the amine-epoxy reaction faster, but using more basic amines simply leads to more carbonation as they will more readily produce blushing via the acid/base interaction. It is interesting to note that in the past when the use of aromatic amine curing agents was more common there was no issue with blushing when using these products because aromatic amines are not basic. Of course, these products are now considered as toxic and as a result are no longer allowed in many parts of the world.
We can see from this that using less basic amines should therefore lead to improved blushing resistance, but they will also produce slow hardeners with long drying times. The question then becomes, how do we produce hardeners which will give the best combination of drying times and resistance to blushing? This is particularly relevant for solvent free systems where we have no drying advantage from the use of solid binders. Curing agent manufacturers have over time developed curing agents with improved resistance to blushing using various formulating methods. For example, forming amine adducts will improve the performance against bushing because the adduction process will remove some of the more basic amine functionality in the curing agent which is often responsible for blushing. It will also improve the compatibility of the amine with the resin thus reducing migration of free amine to the surface of the coating. Advances in curing agent development mean that today the best performing hardeners for blushing resistance in topcoats are based on formulated adducts of one or more amines.
Of course, epoxy resin and curing agent manufacturers can develop and test their resins and hardeners in combination as clear coatings, but the final paint will be formulated by coatings manufacturers where the fillers and additives used will also have an effect on the carbonation resistance of the end product. Even with the recent advances in curing agent performance, the end result will still depend on correct mixing and application of epoxy systems on site ensuring that the conditions in which the coating is applied meet with the manufacturers recommendations.
Stuart Darwen, CTP Advanced Materials GmbH, Germany

Ask the Expert
The questions in this issue relate to painting galvanized steel and predicting CO2 corrosion in oil & gas upsteam operations.
Question:
How do you prepare galvanized steel before it is painted with an epoxy? PS
Answer:
Painting galvanized steel is quite simple as long as the correct steps are used. There are basically four alternative methods to prepare this surface for painting, T-Wash, etch priming, sweep blasting, and weathering. Ideally the surface should be treated immediately after galvanising, but if this is not possible then it can still be carried out later, although the surface must be thoroughly cleaned to remove all contaminants.
T-Wash is a phosphoric acid solution with a small amount of copper carbonate which reacts with the zinc surface and turns it black. An even black colouration confirms that the whole surface is free from grease, and etched ready for painting. The solution must be allowed to dry fully and should be painted as soon as possible, and withing 4 weeks. It should not be used on galvanizing that has been allowed to weather however. There are also other equivalent proprietary products.
Etch primers are similar to T Wash, in that they etch the surface ready for painting, however they have a major disadvantage over T-Wash in that there is no colour change of the surface, and there is thus no indication that the whole surface has been treated. They are however suitable for use on weathered steel.
Sweep blasting at pressures up to 40 psi can roughen the galvanised surface sufficiently to provide a key for the subsequent paint system without removing too much of the zinc surface. Only a fine copper slag, and not the more common angular iron grit should be used, and care needs to be taken to determine the stand off distance and angle of blast to ensure the optimum surface is obtained. Sweep blasting is also often used in conjunction with a chemical pre-treatment.
Exposing a galvanized surface to the environment is another method for preparing it for painting. This should be for a minimum of 6 months, after which the surface is cleaned with a stiff brush to remove the loosely adhering material, leaving a bright zinc metal surface. The surface is then thoroughly washed and allowed to dry fully before being painted.
In all cases, the paint system should be applied according to manufacturers’ recommendations.
It should also be mentioned, that there are some paints which have been specifically formulated to be applied directly to the galvanised surface, and thus no pre-treatment stage is necessary. BG
Question:
How accurately can you predict CO2 corrosion in upstream operations? BK
Answer:
To do full justice to the question really requires writing a major article and even a book. The European Federation of Corrosion (EFC) publication #23, CO2 Corrosion in Oil and Gas Production Design Considerations is an excellent reference, if a little dated now having been published in 1997. Reading through more recent papers on the subject presented at the annual NACE International Corrosion Conferences can provide an excellent source of current thinking and practice.
Nevertheless, it is useful to address the question in two steps. For a given set of conditions, firstly what is the likelihood of CO2 corrosion occurring; and secondly, if likely, in what form and rate will it occur.
While predictive models have become the immediate go-to especially as the computing power and sophistication of laptops, tablets and increasingly smart phones continues to grow exponentially, together with ready access to the internet, it is useful to take a breath to reflect on what simple rules generally are worth having to hand. Concerning the likelihood of CO2 corrosion occurring, the following rule of thumb is worthy of note:
PCO2 < 7 psi (0.5 bar) Corrosion Unlikely
7 psi (0.5 bar) < PCO2 < 30 psi (2 bar) Corrosion Possible
PCO2 > 30 psi (2 bar) Corrosion
An important additional qualification to the above rule is how the following partial pressure ratios, to take account of the presence of H2S if also encountered, effects the resulting corrosion process:
CO2/H2S > 500 CO2 Dominates
500 > CO2/H2S > 20 Mixed CO2/H2S
0 > CO2/H2S > 0.05 H2S Dominates
From a detailed system design and operating standpoint, and for developing a fit-for-purpose corrosion / integrity management strategy, clearly the above rules provide limited hard engineering insight and basis to work from. However, they provide a quick and simple appreciation of the situation ahead of launching into modelling – the latter is a necessary requirement regardless, but not without its challenges and limitation.
There is no one industry recognised, or accepted standard CO2 corrosion model. Over the years there has been a steady growth in the number models in use, in part driven by several of the major oil & gas companies producing their own in-house developed models. All the models are principally directed at predicting CO2 corrosion of carbon and low alloy steels.
It is also worth recognising here the work over many years of de Waard et al (Shell) for their significant openly published contribution to the understanding and key requirements of building a robust CO2 corrosion model and its sound application. In fact, many of the at least 17 models readily found via an internet / published technical papers search owe much to the leading fundamental and practical insight resulting from the work of de Waard et al. It should be noted that most models are empirical in origin based on lab and/or field data. The most extensive and widely recognised theoretically based CO2 corrosion model, a product of a Joint Industry Project (JIP) funded programme at the Ohio University, forms an integral part of Ohio’s Multicorp corrosion prediction software covering almost all key aspects of internal corrosion of mild steel oil and gas pipelines. If not a member of the Ohio JIP, access to the Mulitcorp software is subject to a user charge.
Ready and free access to the models may be limited but a good starting point readily accessible on the internet is: CO2 Corrosion Rate Calculation Model, NORSOK Standard M-506 Rev 2, June 2005. There are commercial models available at a cost, such as the Wood Group’s Electronic Corrosion Engineer ECETM ® tool designed to assist corrosion engineers through the quantitative estimation of corrosion rates, including CO2 corrosion modelling and prediction, and selection of corrosion-resistant materials; and Broadsword Engineering’s proprietary web-based CO2 corrosion model enpICDATM as part of the technical service they offer.
A comparative review of many of the models was presented in Paper No. 10371, NACE Corrosion Conference 2010, San Antonio, CO2 Corrosion Models for Oil and Gas Production Systems. Whereas Paper No. 05552, NACE Corrosion Conference 2005, Houston, entitled A Prophetic CO2 Corrosion Tool – But when is it to be believed? provides additional related background reading resulting from BP’s development of their model Cassandra.
All the models have their strengths and weakness that will to varying degrees be dependent on the specific application. It would be inappropriate here to advocate use of any one model above the others.
- Understanding the current condition and operating details of a system is equally a critically important step to making a sound choice and subsequent application of model. Some key points to consider are:
- Understanding the origin of the model to be used, and how it addresses
the key factors that will determine the predicted corrosion rate
- Range of CO2 partial pressure and temperature applicable
- How it computes pH and range of applicability
- Flow regime and liquid velocity noting that CO2 corrosion is a mass
transfer controlled reaction
- Presence of potential corrosion hot spots – e.g. bends, dead-legs, and
surface flow disturbances such as pre-existing corrosion, weld beads –
can profoundly affect the form of attack (general versus localised
metal loss)
- Surface fouling/shielding due wax, scale, solids drop-out – can
profoundly affect the form of attack (general versus localised metal loss)
- Effect of liquid hydrocarbon phase wetting
- FeCO3 protective scale formation, its nature and stability – will profoundly
affect the form of attack (general versus localised metal loss)
Additionally
- Presence of H2S – often results in very low general corrosion rates but
increased risk of pitting and few models are strong at predicting the joint
corrosive action and rate in the presence of CO2 + H2S
- Presence of dissolved volatile organic acids (e.g. acetic acid/acetate) –
can significantly increase the actual corrosion rate
- Risk of top-of-line corrosion (usually wet gas systems but also may
effect multiphase lines under stratified flow with a gas space) where
water condensation rate is a key factor; also the risk is exacerbated by the
presence of volatile organic acids and H2S
- Presence of solids leading to erosion-corrosion – usually results in a
synergistic effect on resulting metal loss rate for cabon/low alloy steels
that may result in localised attack
Having given due consideration and attention to the above in selecting a model, which can be further helped by looking for field analogues to draw comparisons with, the predicted rates will generally be acceptable for design purposes – e.g. if carbon steel can be used with or without use of a corrosion inhibitor supported by a specified corrosion allowance as part of the required nominal pipewall thickness. Also, predicted rates can be used in support of conducting corrosion risk-based assessments as part of developing a fit-for-purpose corrosion management strategy. If the form of attack is likely to be localised from, for example, consideration of the above points or from inspection data, it is common to apply an escalation factor – typically a 2 or 3 multiplier – to the predicted base corrosion rate. Operating company guidance documents and practices may detail specific requirements for applying escalation factors. It is also important to recognise that poor operation of corrosion control measures such inhibitor treatment and system cleanliness can adversely affect the underlying value of originally predicted corrosion rates.
Effectively managing corrosion solely based on predicted corrosion rates once a system comes into operation is not recommended no matter how good a model is hailed to be. Modelling should be treated as complementary to having in place a proactive and robust risk-based corrosion monitoring and inspection programme that also provides a feedback loop to enable further improvement of a model.
Finally, can any of the models truly predict mm/y corrosion rates to two or even one decimal place? They may display computed rates to such a level, but this could well be more a function of how the software is written than the actual inherent accuracy of the model. Caution should be exercise when using and quoting model-generated predicted corrosion rates to such decimal place accuracy!
Don Harrop FICorr(Hon) FEFC(Hon), CorroDon Consulting Ltd.
Readers are reminded to send any technical questions for possible inclusion in this column. These should be sent to the editor at, brianpce@aol.com


