This article will look at the Electrochemical Noise Method for corrosion monitoring. Firstly, what are its attractions?
Well, it’s as unintrusive as its possible to get, just using the fluctuations which are produced naturally by the corrosion process to tell us the corrosion rate and also, by looking at the plots, the corrosion processes (the degree to which it affects the item being measured is maybe as little like the effect on the star of using the James Webb telescope to obtain its distance based on the red shift!). Secondly the measurement is quick (a few minutes) and interpretation of results is intrinsically simple. It can operate on battery power and the equipment is portable and with appropriate sensing electrodes it can be used for continuous monitoring. The remainder of this article gives examples of how results can be obtained using the ENM technique, not just from organic coatings, but also from reinforcing bars in concrete, and to screen inhibitors. The inhibitors work is part of an ongoing programme at Nottingham university and the other two pieces of work were done by students in Northampton.
First how does the electrochemical noise arise, and what do the results look like?
Rn= v/ I 1
Where Rn is noise resistance,
v and I are the standard deviations of voltage and current values, respectively, measured during a given time period is given by:
V2 = Vj−Vm)2/(n−1) 2
I 2= Ij−Im)2/(n−1) 3
In equations 2 and 3, Vj is the voltage value measured at the jth time interval, Vm is the mean voltage in the given period of time, Ij is the current value measured at the jth time interval, Im is the mean current in the given period of time, and n is the number of time intervals. [1] For coatings, Rn equates to the DC resistance (Rdc), and EIS (0.1 Hz impedance). [2] There is evidence from this work and others that when there is significant corrosion rate, Rn relates to the value Rp obtained from Linear Polarisation Resistance (LPR). The analysis software creates a value of Rn, and typical ENM data are shown in Figure 1.
The first application of the ENM method described is to an anti-corrosive organic coating system.
The actual Noise arrangement used is known as the single substrate (SS) method, diagrammatically shown in Figure 2. You need two working electrodes for noise measurements, and with the single substrate arrangement you can have these as two areas of painted substrate on the same panel, isolated in lab work, by being contained in cells (but in site work, a dry piece of coated steel with a Calomel electrode in each cell, is adequate). The third electrode (reference) is the panel itself. When you examine an organically coated metal, the noise signal tends to be attenuated by the ionic resistance of the coating, and you end up with that resistance being dominant. In this example, the coating was applied to Q panels, cells were attached, and the coated area within each cell was exposed to 0.1 M chloride solution for several months (with occasional topping up). The ENM results were compared against DC resistance. The results are shown in the Figure 3. As can be seen, some areas of coating started with low values of resistance which tended to drop with time, while others had higher initial values, and these remained high. There was a strong correlation between the Rn values, the Rdc values, and the visual appearance. Also, the effects of thickness and number of coats were investigated. Thickness proved more critical than number of coats, although both were important, the higher the thickness the more protective the coating. The advantage of the electrochemical measurement over visual observation is, a) it tells you what is happening earlier, b) It indicates problems when you cannot see what is going on, c) it can be automated, and d) it gives you a number!
These advantages also apply in the case of the second example, that is measuring the corrosion rate of reinforcing bars in concrete. This is little more complicated to set up. The lab work was designed to be a precursor to taking the equipment out to site so non-glass electrodes were used viz solid-state silver /silver chloride solid electrodes, which worked just as well as Calomel electrodes. There is an ENM arrangement which can be applied to a coating or to concrete, which is the NOCS (No connection to the substrate) arrangement. This offers the attraction of being able to get a result without actually making any connection to the rebar itself. The experimental set up is shown in figure 4 and some typical results in Table 1. This work involved two sets of three bars in mortar, one of which contained no added chloride, and the other of which had 4% added chloride. The results were quite clear, the bars in the 4% were corroding (corrosion rate inversely proportional to Rn) many times faster than the bars in the 0% NaCl, although there was some minor variation between bars. Unlike the coating work, we cannot check these particular samples yet as they are still under test and have not been broken open. However previous published work [3] showed beyond reasonable doubt that the Rn value correlated very well to the subsequently observed corrosion.
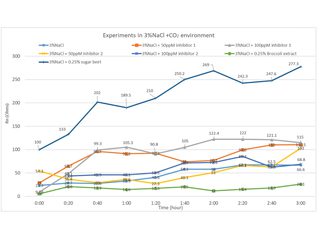
The third example is testing inhibitors This was driven by the wish to test green inhibitors and see if they could be used as alternatives to conventional more toxic inhibitors such as propargyl alcohol. The application considered was the need to inhibit corrosion of the steel pipes used to carry the CO2 saturated oil, containing some sea water. The experimental set up is shown in Figure 5. Two small nominally identical rectangular steel samples were contained in a sample holder made employing the ‘additive manufacturing’ process (a form of 3D printing). In this case it was from an ABS polymer powder. The black blanking-off compound was provided by an anticorrosion coating manufacturer. It was found essential to stir the solution to get reproducible results, although in practise a significant flow rate is likely. Some results are shown in Figure 6, where a particular green inhibitor (sugar beet) proved more effective than conventional inhibitors. The lab investigation compared the ENM method both against corrosion loss by ICP-MS analysis, and also against the more commonly used LPR method. This work has not yet appeared in a journal publication. But a paper has been submitted to EUROCORR 2022.
This has been whistle stop tour showing the application of this ENM technique to three different fields. If anybody wants to find out more, get in touch with the author.
1) Yang, L. and Chiang, K.T. (2010) On-line and real-time corrosion monitoring techniques of metals and alloys in nuclear power plants and laboratories. Understanding and Mitigating Ageing in Nuclear Power Plants., pp. 417-455
2) Comparison of ENM, EIS and DC Resistance for Assessing and Monitoring Anti-Corrosive Coatings Douglas J Mills JCSE 2000
3) Mills, D.; Lambert, P.; Yang, S. Electrochemical Noise Measurement to Assess Corrosion of Steel Reinforcement in Concrete. Materials 2021, 14, 5392. https:// doi.org/10.3390/ma14185392
Acknowledgements
The author would like to acknowledge the assistance of Paul Lambert relation to the concrete project, and extend his thanks to students: Tian Yang Lan, Reuben Osahon and Chiata Collins for permission to publish some of their results. And to DCVG company for providing The Noise Measuring Equipment.
Douglas J Mills
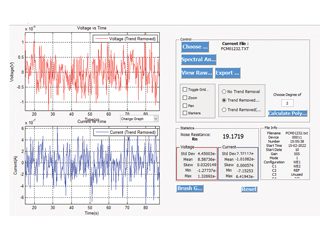
Figure 1: Typically Noise plots of current and voltage against time.
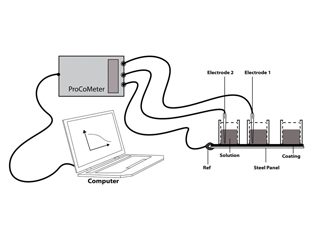
Figure 2: Schematic diagram showing how ENM is applied to coated metal (Single Substrate arrangement) (Solution is typically 0.1 M NaCl).
Figure 3: Typical results obtained from Hammerite coating of Resistance against time.
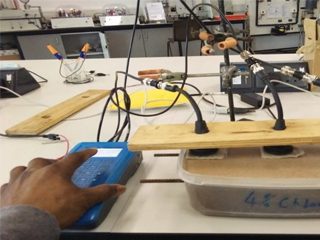
Figure 4: Set up for measuring Rebar in concrete using NOCS arrangement of ENM.